Interim Results
Study Overview
12 MONTH REPORT
Between November 2020 and December 2021, the first QUEST Initiative recruited over 2,300 adult patients in Australia who were new to natural medicine and who suffered from a chronic condition.
Study participants were aged between 18 to 97 years (average age: 51 years) and were 63% female. Participants were recruited across six states by 120 independent doctors, with participants completing a baseline questionnaire before starting natural medicine treatment and then subsequent questionnaires thereafter for 12-months.
The study was supported by health insurance provider Health Insurance Fund of Australia (HIF), guided by an experienced advisory group, and endorsed by a range of national bodies, including MS research Australia, Chronic Pain Australia, Arthritis Australia and Epilepsy Australia.
Results
Summary
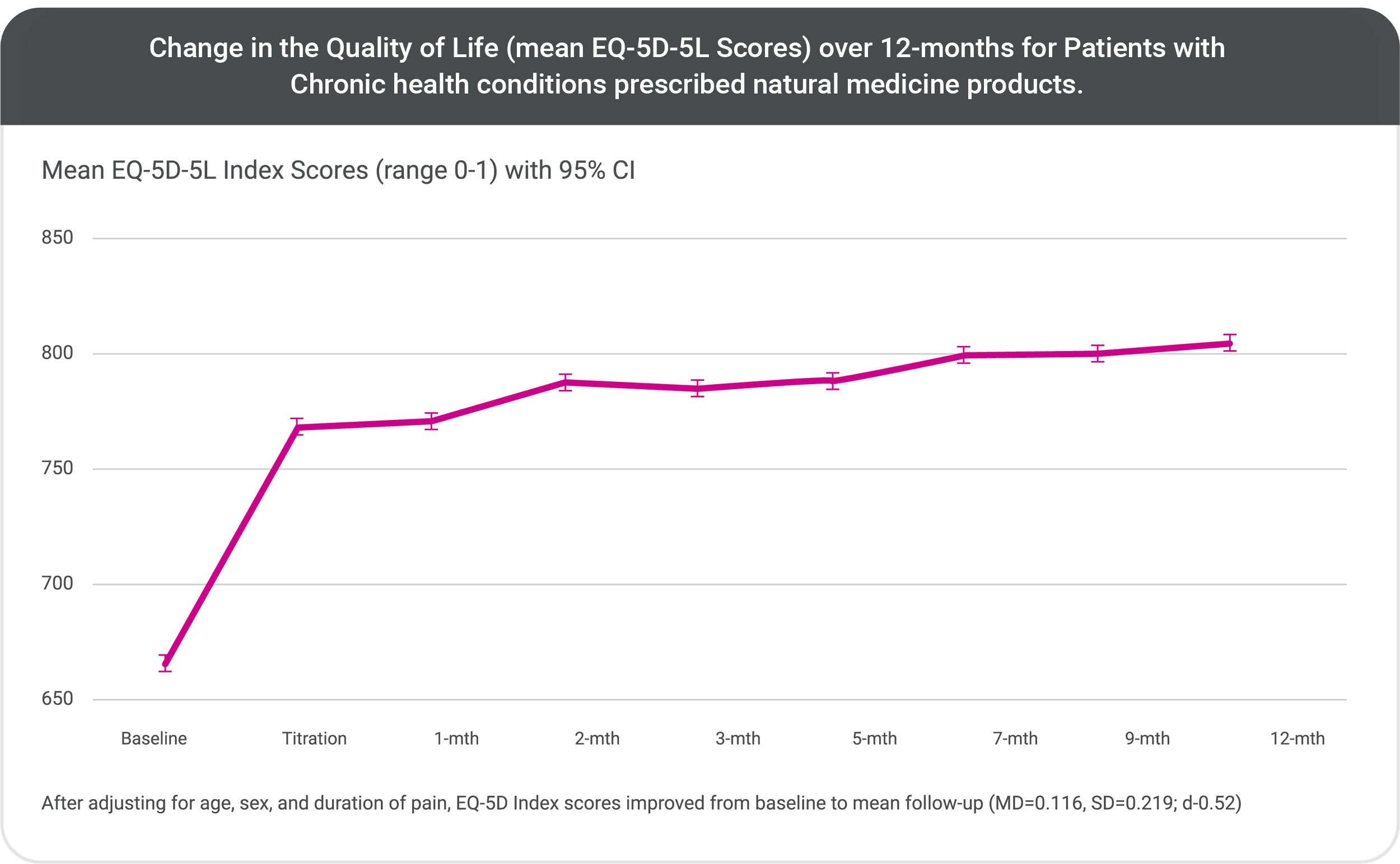
There was a very strong statistical (p<0.001), and meaningful clinical improvement (d=0.52) in the quality of life (QoL) and across a number of chronic conditions for patients receiving natural medicine products.
"Clinically meaningful results" refer to findings that have a significant and important impact on a person's health or well-being that can make a real difference in how healthcare professionals understand or treat a medical condition*.
This rapid initial improvement in QoL was maintained over the 12-months of investigation.
The analysis concluded that it was remarkable for a single medication to exhibit a positive impact on treatment-resistant patients across such a broad range of medical conditions**.
Following the success of the initial QUEST study, the sponsor has launched the next phase of research: The Global QUEST Initiative.
Eligible patients can enrol in this new phase of the study by registering their interest on this website.
* Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003 May;56(5):395-407. doi: 10.1016/s0895-4356(03)00044-1. PMID: 12812812.
** Abelev S, Warne LN, Benson M, Hardy M, Nayee S, Barlow J. MC for the Treatment of Chronic Refractory Pain: An Investigation of the Adverse Event Profile and Health-Related Quality of Life Impact of an Oral Formulation. MCC. 2022 Feb 9;5(1):20-31. doi: 10.1159/000521492. PMID: 35950052; PMCID: PMC9235063.